Mixed results for Moderna mRNA flu vaccine trial
US biotech company Moderna said Thursday it had mixed results from a large-scale trial of its mRNA flu shot, based on the same technology used in its successful Covid-19 vaccine.
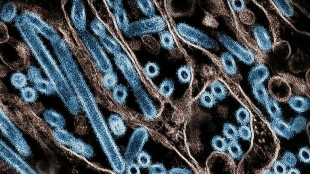
Moderna's experimental mRNA-1010 flu shot is "quadrivalent," meaning it targets four strains of flu: A/H1N1, A/H3N2, B/Yamagata and B/Victoria -- selected based on recommendations by the World Health Organization (WHO).
The Massachusetts-based company said that its flu shot generated an immune response against influenza A strains that was equal or superior to that of already licensed vaccines.
However, it fell short of the already approved vaccines against strains of the less-common influenza B, Moderna said in a statement.
"Today's results represent an important step forward in the development of mRNA-based influenza vaccines," Moderna president Stephen Hoge said.
"We have already updated the vaccine that we believe could improve immune responses against influenza B and will seek to quickly confirm those improvements in an upcoming clinical study."
The Phase 3 trial of the mRNA shot involved 6,102 adults in Argentina, Australia, Colombia, Panama, and the Philippines during the Southern Hemisphere influenza season.
Participants received a single dose of mRNA-1010 or a single dose of a licensed influenza vaccine.
Moderna said 70 percent of the mRNA-1010 recipients reported adverse reactions such as headaches, swelling and fatigue compared to 48 percent in the other group.
Virus strains have to be selected six to nine months before the vaccines are intended to be used, and their efficacy is approximately 40 to 60 percent.
Moderna is simultaneously conducting an efficacy trial of its vaccine.
Moderna and other vaccine manufacturers, including Sanofi, hope that mRNA technology -- which provokes an immune response by delivering genetic molecules containing the code for key parts of a pathogen into human cells -- can accelerate immunization development and production, and heighten efficacy.
There are some three to five million severe influenza cases annually worldwide and between 290,000 and 650,000 deaths, the WHO says.
Moderna's stock price was down more than six percent in after-hours trading in New York.
M.Huber--HHA